Abstract
Background: Common measures and common data elements (CDEs) for sickle cell disease (SCD) are needed to help improve data quality and data comparability necessary for meta-analyses, guidelines development, comparative effectiveness research, and implementation science. In 2014, the National Heart, Lung, and Blood Institute (NHLBI) launched the PhenX (consensus measures for Phenotypes and eXposures) Measures for Sickle Cell Disease Research Project to establish a framework for data sharing across different SCD research projects. As a result of this project, a Core Collection and two Specialty Collections (Cardiovascular, Pulmonary, and Renal; Neurology, Quality of Life, and Health Services) of standard measure protocols were added to the PhenX Toolkit. The PhenX Toolkit (https://www.phenxtoolkit.org/) is a Web-based catalog of standard protocols and associated interoperability resources to help investigators improve quality of data collection and identify opportunities for collaborative research. In 2019, NHLBI provided additional funding to expand the collections of sickle cell disease measurement protocols in the PhenX Toolkit. Under the guidance of a Sickle Cell Disease Research and Scientific Panel (SRSP), six additional collections of protocols for use in SCD research will be added to the PhenX Toolkit. One of these collections of protocols relates to research involving SCD Pain.
Methods: The SCD Pain Working Group (WG), consisting of 10 SCD experts, was convened to select high priority protocols for inclusion in the Toolkit. The SCD Pain WG selected representative protocols available in published literature using a consensus process which included input from the scientific community. Protocols were selected based on availability, protocol burden, and validity, with emphasis on measures being well established and broadly validated. Several included protocols had been previously validated in populations with SCD. For each measure, the Toolkit provides a description of the measure, the rationale for its inclusion, detailed protocol(s), and supporting documentation. The Toolkit also provides data collection worksheets and data dictionaries to help integrate PhenX measures into their study design. To support investigators who want to collect data via the Web, PhenX protocols are available as REDCap Instrument Zip files.
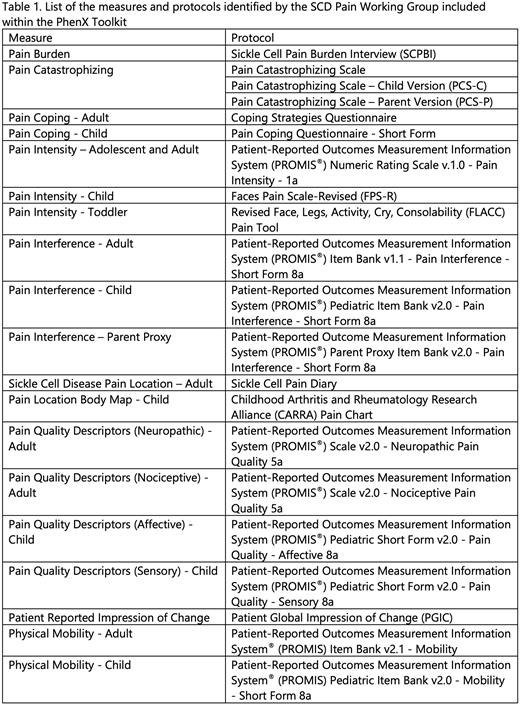
Results: The SCD Pain collection of protocols was released into the Toolkit (https://www.phenxtoolkit.org/sub-collections/view/28) in May 2022. The SCD Pain WG identified a set of 23 protocols that encompass the burden of pain on an individual's day-to-day life, the magnitude of pain experienced by an individual, the location of the pain experienced, the extent to which the pain interferes with the person's daily life, the individual's overall physical mobility, the specific subjective sensations associated with pain, and the coping strategies employed by the individual (Table 1). For many of the measures, separate adult and child protocols were identified. These protocols, including the process, criteria, and rationale for their selection, will be presented.
Implications: Consistent use of these protocols in future clinical and epidemiologic studies will allow clinicans and researchers to quantify how SCD impacts a patient's everyday life and develop more effective interventions. For researchers, adoption and use of PhenX protocols will facilitate cross-study analysis domestically and internationally, accelerate translational research, and lead to greater understanding of SCD pain phenotypes. For clinicians, using PhenX measures in routine clinical practice will help improve patient care and quality of life. Consistent use of these standard measures will establish a common currency to help better understand the etiology, progression, and treatment of SCD. Funding was provided by a Genomic Research Grant (U41HG007050) from NHGRI, with current or prior funding support from the National Institute on Drug Abuse (NIDA); Office of Behavioral and Social Sciences Research (OBSSR); National Institute of Mental Health (NIMH); National Heart, Lung, and Blood Institute (NHLBI); National Institute on Minority Health and Health Disparities (NIMHD); and Tobacco Regulatory Science Program (TRSP).
Disclosures
Guarino:Novartis: Consultancy. Darbari:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Kenney:Global Blood Therapeutics: Research Funding. Smith:Novo Nordisk: Other: DSMB; Novartis: Consultancy, Honoraria; Global Blood Therapeutics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Emmaus: Consultancy; Forma Therapeutics: Consultancy, Research Funding; Agios: Research Funding; Pfizer: Consultancy, Research Funding; Imara: Research Funding. Stinson:Pfizer: Other: Unrestricted grant. Zempsky:Lundbeck Pharma: Consultancy; GSK: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal